Trichlormethiazide
- B (D if used to treat pregnancy-induced hypertension)
administration
- C03AA03 (WHO)
- In general: ℞ (Prescription only)
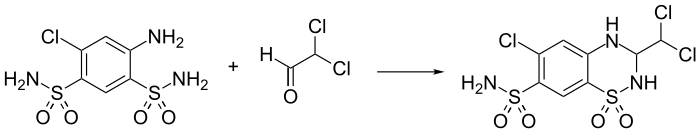
- 6-Chloro-3-(dichloromethyl)-1,1-dioxo-3,4-dihydro-2H-benzo[e] [1,2,4]thiadiazine-7-sulfonamide
- 133-67-5
 Y
Y
- 5560
- 7314
- DB01021
 N
N
- 5359
 N
N
- Q58C92TUN0
- D00658
 N
N
- ChEMBL1054
 N
N
- DTXSID7023699

 N
N Y (what is this?) (verify)
Y (what is this?) (verify)Trichlormethiazide (INN, currently being sold under the brand names of Achletin, Diu-Hydrin and Triflumen) is a diuretic with properties similar to those of hydrochlorothiazide.[1] It is usually administered for the treatment of oedema (including that which is associated with heart failure, hepatic cirrhosis and corticosteroid therapy) and hypertension.[1] In veterinary medicine, trichlormethiazide can be combined with dexamethasone to be used on horses with mild swelling of distal limbs and general bruising.[2]
As a diuretic (in particular a thiazide), trichlormethiazide encourages water loss from the body.[1] Trichlormethiazide works by inhibiting Na+/Cl− ion reabsorption from the distal tubules of the kidneys.[1] In addition, trichlormethiazide increases the excretion of potassium.[1]
Mechanism
Trichlormethiazide appears to block the active reabsorption of chloride and possibly sodium in the ascending loop of Henle. This results in excretion of sodium, chloride and water, and thus acts as a diuretic.[1] Although trichlormethiazide is used to treat hypertension, its hypotensive effects may not necessarily be due to its role as a diuretic.[1] Thiazides in general cause vasodilation by activating calcium-activated potassium channels in vascular smooth muscles and inhibiting various carbonic anhydrases in vascular tissue.[1]
Synthesis
References
- ^ a b c d e f g h "DrugBank: DB01021 (Trichlormethiazide)". DrugBank. Retrieved 2008-01-23.
- ^ "Trichlormethiazide and Dexamethasone for veterinary use". Wedgewood Pharmacy. Retrieved 2008-01-24.
- ^ GB 949373, "Benzthiadiazine derivatives and processes for their manufacture", published 1960, assigned to Scherico Ltd.
- ^ DE 1147233, de Stevens G, Werner LH, "Verfahren zur Herstellung von 2-Alkenyl-7-sulfamyl-3, 4-dihydro-1, 2, 4-benzothiadiazin-1, 1-dioxyden", published 1960, assigned to Ciba Geigy
- ^ De Stevens G, Werner LH, Barrett WE, Chart JJ, Renzi AH (March 1960). "The chemistry and pharmacology of hydrotrichlorothiazide". Experientia. 16 (3): 113–4. doi:10.1007/bf02158094. PMID 13815073. S2CID 35063235.
- ^ Sherlock MH, Sperber N, Topliss J (May 1960). "3-Haloalkyl-dihydrobenzothiadiazine dioxides as potent diuretic agents". Experientia. 16 (5): 184–5. doi:10.1007/BF02178974. S2CID 3086415.
- ^ GB 954023, "Novel process for preparation of dihydrobenzothiadiazines", published 1960, assigned to Scherico Ltd.
- ^ US 3264292, Close WJ, published 1960, assigned to Abbott Labs
- v
- t
- e
(and etacrynic acid)
| CA inhibitors (at PT) | |
|---|---|
| Loop (Na-K-Cl at AL) | |
| Thiazides (Na-Cl at DCT, Calcium-sparing) | |
| Thiazide-likes (primarily DCT) |
| ESC blockers | |
|---|---|
| Aldosterone antagonists |
(DCT and CD)
- #WHO-EM
- ‡Withdrawn from market
- Clinical trials:
- †Phase III
- §Never to phase III
 | This drug article relating to the cardiovascular system is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e










