Plicatin B
 | |
| Names | |
|---|---|
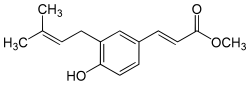
| Preferred IUPAC name Methyl (2E)-3-[4-hydroxy-3-(3-methylbut-2-en-1-yl)phenyl]prop-2-enoate | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL |
|
| ChemSpider |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C15H18O3 |
| Molar mass | 246.30 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) N ?) Infobox references | |
Chemical compound
Plicatin B is a hydroxycinnamic acid found in Psoralea plicata.[1]
References
- ^ Plicatin A and B, two phenolic cinnamates from Psoralea plicata. Nazli Rasool, Abdul Qasim Khan and Abdul Malik, Phytochemistry, Volume 29, Issue 12, 1990, Pages 3979-3981, doi:10.1016/0031-9422(90)85385-S
- v
- t
- e
Types of hydroxycinnamic acids
| Precursor |
|
|---|---|
| Monohydroxycinnamic acids (Coumaric acids) |
|
| Dihydroxycinnamic acids |
|
| Trihydroxycinnamic acids |
|
| O-methylated forms | |
| others |
|
| glycoside-likes |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tartaric acid esters |
| ||||||||
| Other esters with caffeic acid |
| ||||||||
| Caffeoyl phenylethanoid glycoside (CPG) |
|
| Dimers |
|
|---|---|
| Trimers |
|
| Tetramers |
|
coenzyme A (CoA)
- Caffeoyl-CoA
- Cinnamoyl-CoA
- Coumaroyl-CoA
 | This article about an aromatic compound is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e











